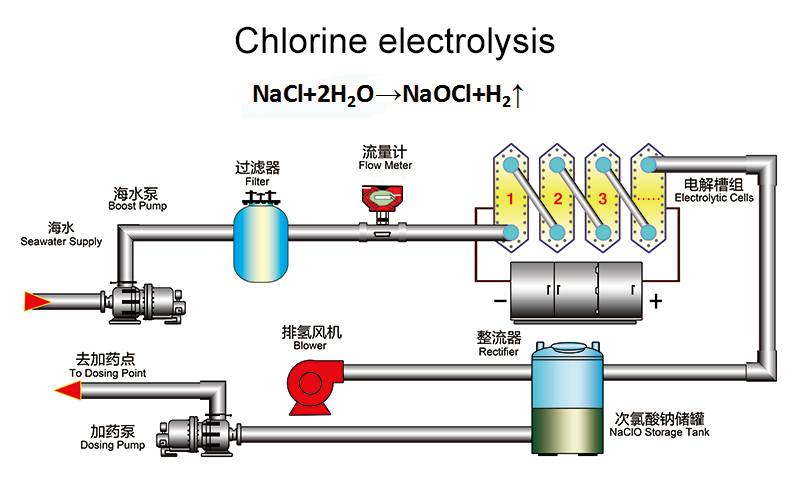
Total salinity of seawater is about3~4%.The content of sodium chloride is about 2.5%. Seawater of constant flow rate is injected into the cell without the diaphragm electrode structure. The electrolyzer is energized the direct current. Because sodium chloride in seawater exists ionic condition. In the effect of the electric field, anode’s surface produces Cl2. Cathode’s surface produces H2. Cl2 and NaOH will have secondary chemical reactions in solution and produce NaClO. The reaction equation is:
Ionization reaction: NaCl="Na++Cl~;H2O=H++OH~"
Electrochemistry Reaction:
Anode reaction: 2Cl-→Cl2+2e
Cathode reaction: NaCl+H2O→NaClO+H2↑
Chemical reaction in solution: Cl2+2NaOH→NaCl+NaClO+H2O
Qingdao Laoshan District, Zhuzhou Road, 149-1
Phone:86-532-68725725
E-mail:sunrui@sunrui.net
| Name: | |
| Feedback | |